There’s a way to cut 80% of Cell-Ag costs
Today, we consume all sorts of abstractions. Beef patties look nothing like cows. Sausages look nothing like pigs. Chicken nuggets look nothing like chickens.
The truth is, we don’t need the full animal for just the meat. With the emerging field of cellular agriculture, it is now possible to grow meat in a lab with a couple of cells taken harmlessly from the animal.
Yet, as of now, lab-grown meat is still unethical. There is one huge bottleneck that’s responsible for 80% of the cost, technical challenges, and the killing of unborn fetuses.
The growth medium.

Here’s a breakdown of how lab-grown meat is made:

We start with myosatellites (aka muscle stem cells), the precursors to muscle cells.
But why do we need to use myosatellites? Why can’t we start with muscle cells?
That’s because muscle cells are unable to undergo mitosis and divide. They must rely on myosatellites to proliferate.
Now that we have the myosatellites, we have to make them feel safe enough and have the necessary resources to grow. We do this by submerging them in growth media, a liquid broth of nutrients that convinces them that they’re actually inside a cow instead of in some unfamiliar petri dish with scientists gazing over them.
Most of the media consist of amino acids, sugars, and vitamins — quite simple to make, not dissimilar to gatorade🥤. However, 10–20% of the media is animal serum, commonly fetal bovine serum (we’ll get to why in a bit). This animal serum contains the integral growth factors, standard proteins, and hormones for myosatellites to grow.
In this growth media, the myosatellites differentiate into the muscle cells that we’re used to eating.
Moving on… to the scaffold. When building a house, we first need a scaffold to construct everything else in a neat and orderly fashion. Likewise, for cultured meat, we need a scaffold to help the cells develop into a 3D structure and not a floppy layer of meatiness.
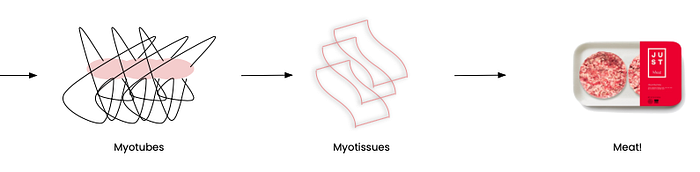
With everything in place, the myosatellites are set to complete their life mission, proliferating into myotubes, myotissues, and finally real meat!
But hold up, rewind back to the growth medium part. Why is it so unethical and expensive to produce?
To understand, we need to start from where FBS is first obtained — the slaughterhouse.
The Origins of FBS
Fetal bovine serum is derived from the blood of cow fetuses (more specifically, it’s the blood without cells, platelets and clotting factors). Cow fetuses are derived from pregnant cows.
In the slaughterhouse, if a cow is discovered to be pregnant, its reproductive tract is dropped down a stainless-steel chute leading to the calf-processing area. There, the calf is removed from the uterus, cleaned and a cardiac puncture is performed.
A cardiac puncture is when a needle is inserted directly into the heart of an unanaesthetised fetus and its blood is extracted… ouch. To obtain an adequate FBS harvest, the fetus has to be alive through everything because blood coagulates immediately upon death.
The blood is left to clot at a low temperature and the serum is separated from the rest of the blood by refrigerated centrifugation 🌪. The rest of the fetus is processed for animal feed and the extraction of specific fats and proteins.

The amount of serum that is obtained depends on the size and age of the fetus. A 3-month-old fetus yields about 150ml of raw FBS, at 6 months, 350ml can be obtained, and a 9-month-old fetus (near-term) gives 550ml.
In 2016, 700 000 litres of FBS were produced, meaning that around 2 000 000 fetuses had to have undergone the serum collection process.

Yet the problems don’t end there…
A Huge Technical Issue with FBS — Unreliability
Because of variations in worldwide livestock numbers, trade regulations, beef and dairy prices, livestock feed costs, and weather conditions, the price and availability of FBS fluctuate. As with everything else in biology, it’s not like labs can simply order FBS on-demand with same-day shipping.
Once labs finally do receive the serum, it still cannot be immediately used. A portion has to be tested for batch-to-batch variations. Cows around the world are not raised in perfectly identical conditions. Different FBS batches can have varying concentrations of growth factors which then lead to inconsistencies in the cultured meat.
Altogether, low global supply, unreliability, and high volume requirements (increased demand) of FBS results in a single litre costing at least $100USD. 100s of litres of FBS are required to produce 1kg of beef — equivalent to 20 McDonalds hamburgers.
No one will want to pay $500 for a hamburger if they can get one for 100x less.
Clearly, using natural FBS is not sustainable or scalable. However, sticking to traditional agriculture methods isn’t either.
A few days ago, I went out west of the GTA with some friends to talk to some cash crop farmers and gain their perspectives on our iGEM project.
On and on we drove, passing by acres of cornfields, until stopping at a wild-west-themed corner shop. We stepped in and asked the clerk if there were any corn farmers in the area.
Her answer — “You’ll probably have to travel to Guelph, all the corn out here is for cows.” For context, Guelph was 55km away. It would take us an hour to get there by car.
And that’s when it really hit me that cows — and livestock in general, are a real problem affecting the world right outside my city. Based on some rough calculations, in that area alone, approximately 40 million ears of corn were grown that would never fulfill their life mission of sustaining a human because they would be consumed by a cow… who would then be eaten by a human anyway. But, cows use 90% of their food for bone formation, blood formation, brain formation, and muscle formation. So if we feed a cow 26 ears of corn, we would only be getting 2.6 ears back when we actually eat the cow.

Not only are cows ripping us off, but they’re also saying RIP to the environment because:
- Animal agriculture is responsible for 18% of all greenhouse gases (cars & other modes of transportation are responsible for 13%). Animals are also more potent than cars because they emit methane as a waste product. Methane has a global warming potential 86x that of CO2. [Source]
- We’re in a global water crisis. Only 1% of all the water in the world is drinkable, yet 70% of that available fresh water worldwide is used for livestock consumption, irrigating livestock feed, and cleaning the livestock and equipment for human consumption. [Source]
- Land for livestock + land used to grow the food that livestock eats takes up 39% of habitable land worldwide. And it’s not acres of rolling green pastures either because 99% of meat production happens in largely unethical factory farms. [Source]
Quick recap: traditional animal agriculture is terrible for both our environment and economy, but cultured meat faces 1 major bottleneck — the unethical and unreliable growth medium, responsible for 80% of total production costs.
So you may be thinking, why don’t we just create a better growth medium and have lab-grown meat take over the world?
Enter Multus. Armed with the intelligence of ML and the science of yeast engineering, Multus is creating a completely animal-free growth media with the key growth factors found in fetal bovine serum, but at 1% of the cost and 2x the lifespan.

I got to interview Cai Linton, CEO of Multus to learn more about how his company is making the impossible inevitable. Interesting insights below.
Multus in 6 words: creating ingredients to scale cultured meat.
Q: Why hasn’t synthetic FBS been made yet if it’s such a huge issue?
FBS works really well. FBS is like a one-size-fits-all solution. It contains a cocktail of proteins that make it ideally suited for helping all kinds of animal cells grow and duplicate. If you need a replacement, you’d develop a specific formulation for a specific cell. This makes it super expensive and not very useful if you’re looking to grow a variety of cell types.
Multus is creating the world’s first universal FBS replacement to give high performance across a number of different variants of cell lines. This universal formulation will maximize impact by catalyzing as many companies as possible to develop new technologies and push their products out of the lab into pilot-level production.

Side note: the M in Proliferum M stands for mammalian, meaning that it is tailored to support the growth of mammalian cells only. However, Multus is also planning to design for formulations for avian cells (chicken, duck) as well as seafood.
Q: There are also other companies in the race to develop a synthetic FBS, what differentiates Multus? (pun not intended)
To develop the FBS formulation, Multus takes a data-driven approach to find which ingredients are very useful in growth and in which proportions they work best. This top-down system is very standardized and can work with many different cell types, whereas a lot of other companies are designing media formulations for only a small subset of cells.
But there’s more.
Multus is not only optimizing the recipe, but also the ingredients themselves.
Natural growth factors are very thermosensitive and break down within 2–3 days. Multus is using protein engineering to design better growth factors to last 2x longer, cost 99% less and be ultra-reliable.
You can think of developing an animal-free FBS like baking a cookie 🍪. Not only is Multus curating the most fool-proof recipe, but they’re also crafting the sweetest sugar, freshest butter, etc.
A bit of backstory: Around a year ago, Multus joined Indiebio’s NY 01 cohort. Indiebio is a biotech accelerator, but it’s also the birthplace of the cultivated meat industry. Back in the day when biotech was largely tied to therupeutics and researchers were not seen as businesspeople, Indiebio was the only place where innovators with wild visions of the future of food could go. The result? Companies such as Clara Foods, Geltor, Upside Foods, NotCo, Fineless Foods and now, Multus.
Q: What was one of your biggest lessons learned from Indiebio?
Getting critical feedback from believable people is one of the best things you can do to supercharge your success. When building a company as a bunch of researchers, there are a lot of questions that come up and a lot of things that you have to do but don’t necessarily know why or don’t necessarily know if you’re doing a good job of it.
So you try different ideas and then for somebody to give their honest feedback is really useful because investors or customers won’t go to you and say something like, oh your slides look terrible, or this part doesn’t make sense.
Implementation is 99% of the work. Ideas are pretty cheap. Get personalized feedback on implementation.
Q: What was the biggest challenge in turning Multus from an idea into a successful company?
Mainly creating a product that people will pay for (finding a product-market fit). Specifically for Multus, we’ve jumped between just focusing on growth factors and focusing on whole media. Through conversations with potential customers, we realized that we can bring far more value by looking at the entire challenge of growth media, not just tackling the cost element, but also looking at performance with how the ingredients come together and how all of our inputs can scale as our customers go to market.
Another is having the right direction. When creating a company from scratch, there are a million different directions you can go into. But you need to focus your attention on moving the business and product forward. This means saying no to a lot of things, which is difficult because you want to keep as many options open as possible. Consider many opinions but oftentimes it’s best to do one or two things very well before jumping to the next step.
Q: How can you (reading this) help Multus?
Be passionate. Train in the right areas of science and engineering. Focus your attention on tackling these grand challenges. Be part of the good fight and get involved. I think that’s the biggest thing, and we’d love to have you join us at Multus in a couple years time.
Q: Do you have any advice for people who want to make an impact in the cell-ag industry but don’t know where to start?
Try and make yourself useful. Even if you don’t think you can get a full internship at a company, if you can identify a problem, think of how you might solve it, and then say, look, I noticed this is perhaps a problem you’re facing in your company. I’d love to work with you to solve it. This is how I think I’ll do it if you let me work at your company for five weeks.
Here are some awesome articles written by my friends if you want to dive deeper into cell-ag :
- Kimberly Liang writes about optimizing bioreactors and using 4D scaffolding to improve cultured meat production.
- Isabella Grandic writes about consumer uptake, hurdles to and the chemistry behind in-vitro meat production.
- Okezue Bell writes about milk without the moo, fish without the fin, and much more on the future of food.
The Future that Multus is Building
It’s time for a global mindset shift from slaughter culture to cell culture.
Multus has paved the path for the end-to-end cultivated meat process to be completely animal-free. With Multus, leading cultivated meat companies can now profitably scale-up, and entry barriers will be lowered for new innovators to drive this space forward.
Multus empowers the cultivated meat landscape to take a bigger slice of the >$1 trillion USD global meat industry and with it reduce its huge environmental footprint.
Compared with traditional meat production, clean meat uses 100x less water eliminates the need for antibiotics, and reduces greenhouse emissions and land use by the same amount.
Learn more about Multus here: Website | Promo Video | Pitch | Founder Story
Thanks for making it to the end of this article! I’m Amy, a 15 y/o synbio researcher who loves all things bio. If you’d like to stay in touch, feel free to subscribe to my monthly newsletter, connect with me on LinkedIn, or follow me on Twitter :) Have an awesome day!
